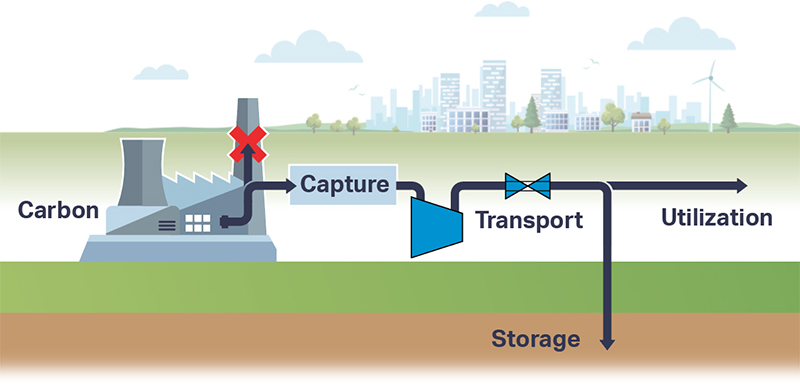
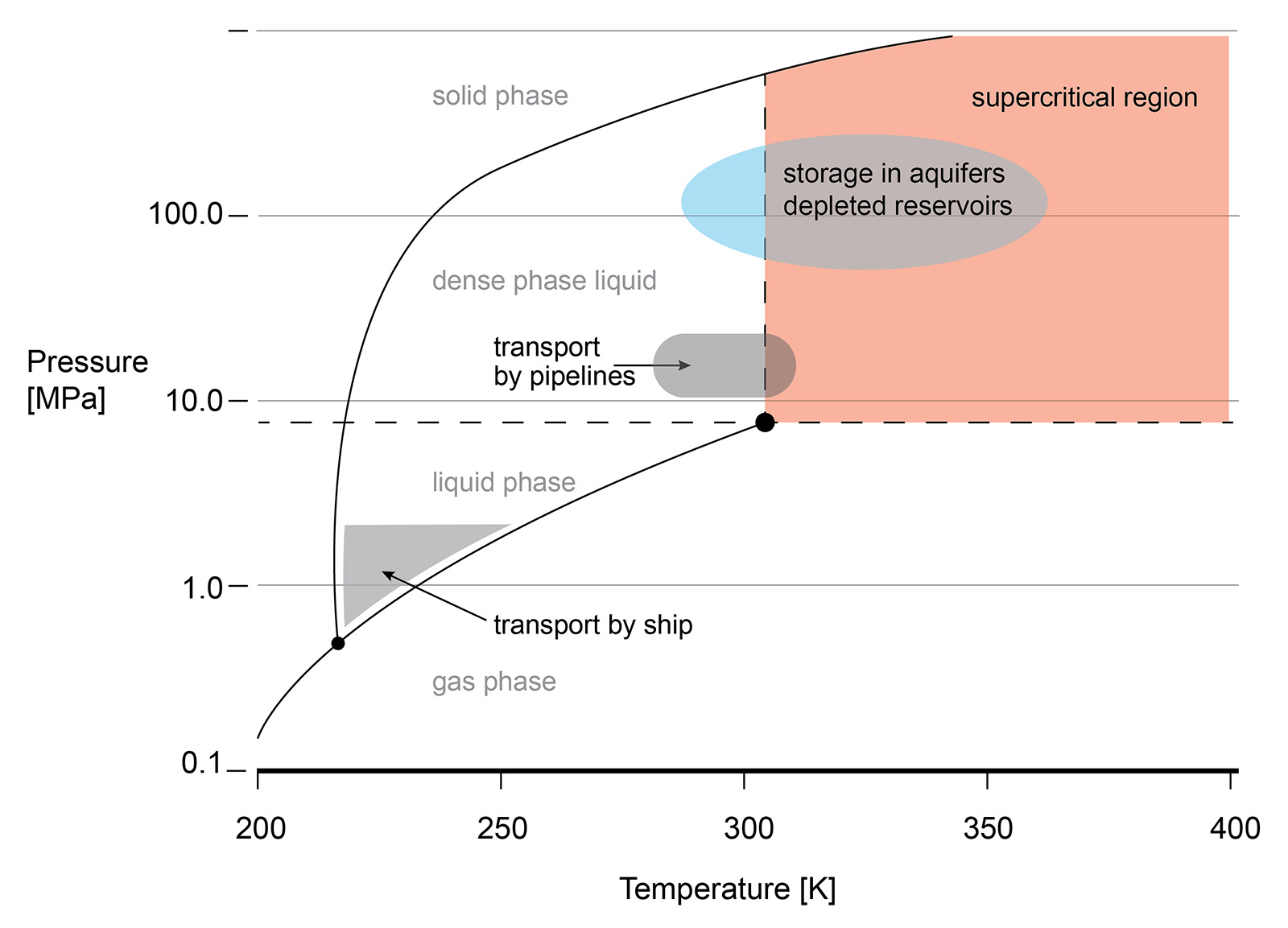
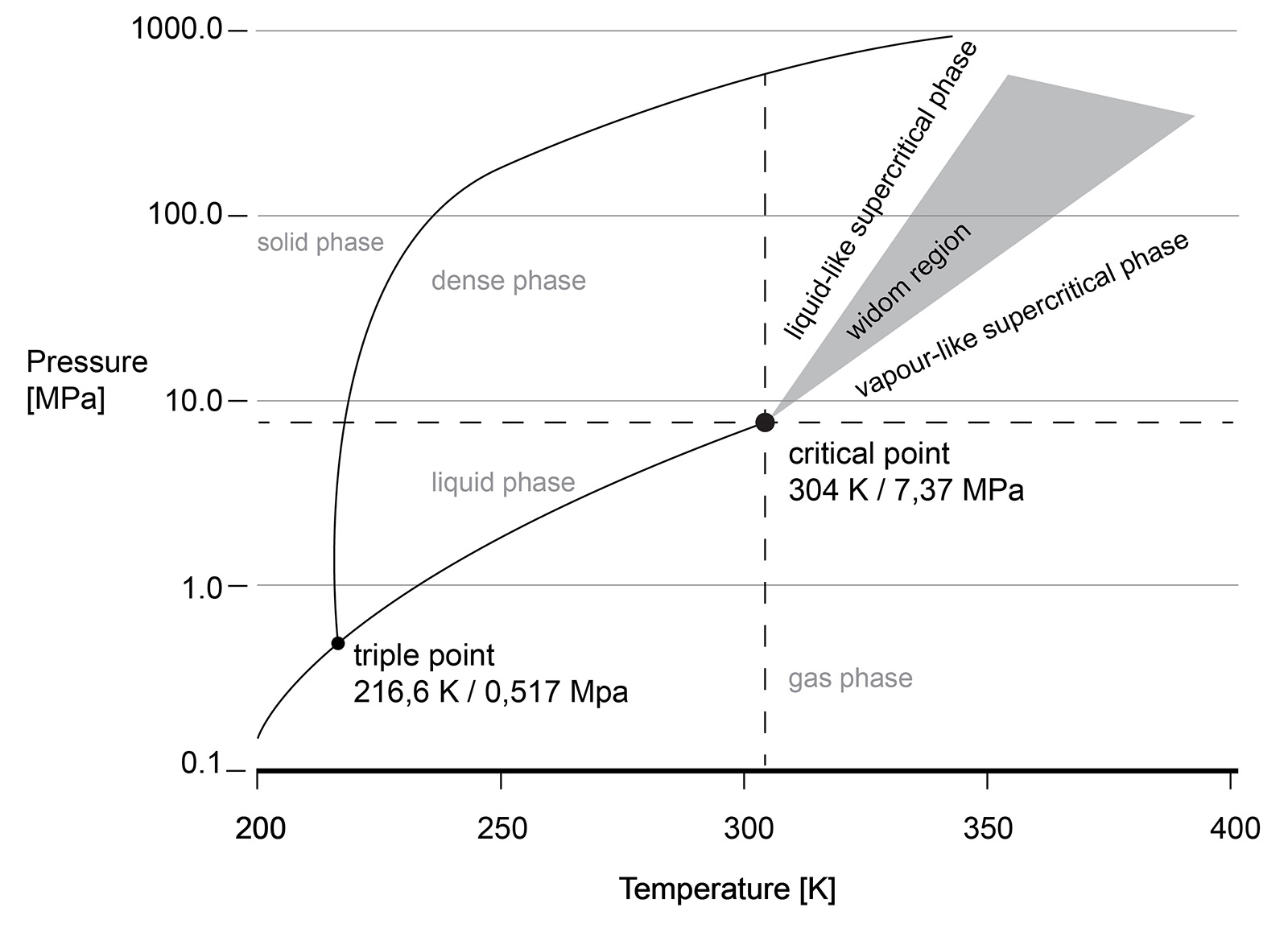
The supercritical state of CO2 occurs at a low pressure and temperature (73.8 bar, 31.3 °C). sCO2 behaves like a compressible fluid, however with a density of a liquid. Close to the critical point, known as the Widom area, the fluid exhibits large anomalies in behaviour. necessitating valve outlet conditions to be evaluated and taken into account during valve sizing and selection. Phase changes, Joule-Thomson effect, can result in multiphase flow, CO2 clathrate formation (similar to hydrate formation in natural gas production) and possibly even dry ice depending on impurities in the gas.
To properly size a valve and select the correct Cv and cage type, it is of utmost importance that the correct fluid properties at the inlet as well as the outlet of the valve are used. This includes values upstream and downstream for temperature, density, compressibility and k-factor (cp/cv).
The axial flow concept, where the flow enters the pipe line directly after exiting the cage and thus avoiding extra turbulences is a good solution.
Understanding the phase diagram and fluid behaviour under supercritical or dense conditions is required for the best valve selection. For example, the vast changes in density are the reason that valves with cavities, where the fluid can be 'trapped', are best avoided. Changes in the pressure or temperature within the cavity may result in a change of the fluid density and lead to excessive pressures in the cavity.